Der Futtersaft der Honigbienen
Honigbienen füttern ihre Larven nicht direkt mit dem gesammelten Nektar oder Pollen, sondern die jungen Honigbienenarbeiterinnen (auch Ammenbienen genannt) produzieren daraus Futtersaft (Swammerdam, 1738). Dies geschieht in den Hypopharynx- und Mandibeldrüsen, welche sich im Kopf der Arbeiterinnen befinden (Kratky, 1931; Schiemenz, 1883). Futtersaft ist also ein Drüsensekret. Alle Honigbienenlarven bekommen erstmal grundsätzlich diesen Futtersaft (von Planta, 1888; 1889); aber je nachdem, ob sich die Larve dann mal zu einer Arbeiterin, Königin oder Drohne entwickelt, unterscheidet sich der Futtersaft ein wenig in seiner Zusammensetzung; vor allem aber unterscheidet sich die Menge an Futtersaft, die die verschiedenen Larven erhalten (Snodgrass, 1925; von Planta, 1888, 1889; Wang et al., 2016). Futtersaft, der an Königinnenlarven verfüttert wird, wird auch Gelée Royale genannt (Huber, 1792).
Der Futtersaft enthält nun alle möglichen Nährstoffe, die Honigbienenlarven so brauchen; unter anderem auch Proteine, die von den Drüsenzellen der Hypopharynxdrüse synthetisiert und sekretiert werden (Patel et al., 1960). Anfang der 1990er Jahre wurde das Hauptprotein aus Gelée Royale isoliert und Haupt-Gelée-Royale-Protein (auf Englisch major royal jelly protein = MRJP) genannt (Hanes & Šimúth, 1992). Nun enthält Gelée Royale aber nicht nur dieses eine Protein, sondern mehrere (siehe Abbildung); und man stellte im Laufe der Zeit fest, dass fast alle diese Proteine eine ähnliche Aminosäuresequenz aufweisen und somit zu einer Proteinfamilie, der Familie der Major Royal Jelly Proteine (MRJPs), gehören. Aufgrund des hohen Vorkommens von essentiellen Aminosäuren in diesen Proteinen wurde früher angenommen, dass die Proteine während der Larvalentwicklung hauptsächlich eine Ernährungsfunktion haben (Albert et al., 1999a, 1999b; Schmitzová et al., 1998).
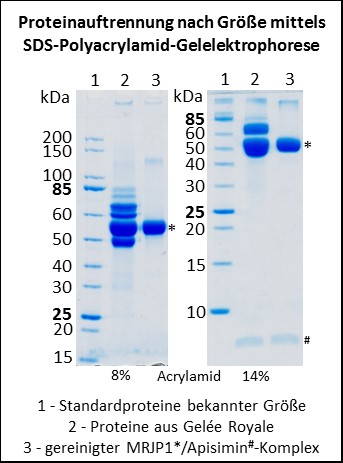
Mit der Veröffentlichung des Genoms der Westlichen Honigbiene war dann klar, dass Apis mellifera insgesamt neun Gene besitzt, die für MRJPs kodieren, und die Gene wurden einfach von eins bis neun durchnummeriert (Weinstock et al., 2006). Das 1992 erstmalig isolierte Haupt-Gelée-Royale-Protein hat die Nummer eins erhalten und heißt nun MRJP1. Interessanterweise liegen alle neun mrjp Gene hintereinander auf dem gleichen Chromosom und sind wahrscheinlich durch Genduplikation entstanden (Drapeau et al., 2006). Alle anderen bisher sequenzierten Bienenarten, z.B. die Dunkle Erdhummel Bombus terrestris und die Luzerne-Blattschneiderbiene Megachile rotundata, besitzen nur ein einziges MRJP (Kapheim et al., 2015; Sadd et al., 2015).
Wie man nun nach dieser Einleitung unschwer erraten kann, dreht sich meine Forschung um diese MRJPs. Mich interessiert vor allem, was die Funktionen der neun MRJPs in Apis mellifera sind, warum die anderen Bienenarten nur ein einziges MRJP besitzen und was die Funktion dieses einen MRJPs ist.
Der Komplex aus MRJP1 und Apisimin
Obwohl man mittels Massenspektrometrie alle neun MRJPs im Gelée Royale nachweisen kann (Schönleben et al., 2007; Zhang et al., 2014), machen MRJP1, 2 und 3 den Hauptanteil der Proteine im Gelée Royale aus (Schmitzová et al., 1998). Reinigt man MRJP1 aus Gelée Royale auf, dann geschieht das häufig zusammen mit dem verhältnismäßig kleinen Protein Apisimin (circa 5 kDa) (Bíliková et al., 2002). Das liegt daran, dass MRJP1 und Apisimin sich gegenseitig binden und einen Proteinkomplex ausbilden (Mandacaru et al., 2017; Tamura et al., 2009; Tian et al., 2018). Die Funktion dieses Komplexes war bisher nicht bekannt. Wir konnten nun zeigen, dass der Komplex, der bei einem neutralen pH von 7.0 aus vier Molekülen MRJP1 und aus vier Molekülen Apisimin besteht (Mandacaru et al., 2017; Tian et al., 2018), bei einem pH unterhalb von 5.0 anfängt fibrilläre Strukturen zu bilden (Buttstedt et al., 2018). Bei pH 4.0, dem natürlichen pH von Gelée Royale, bilden die beiden Proteine dann wunderschöne Proteinfibrillen. Zudem konnten wir zeigen, dass auch Gelée Royale unter dem Elektronenmikrsokop viele fibrilläre Strukturen aufweist, die zerstört werden, wenn man den pH künstlich auf pH 7.0 erhöht (Kurth et al., 2019).
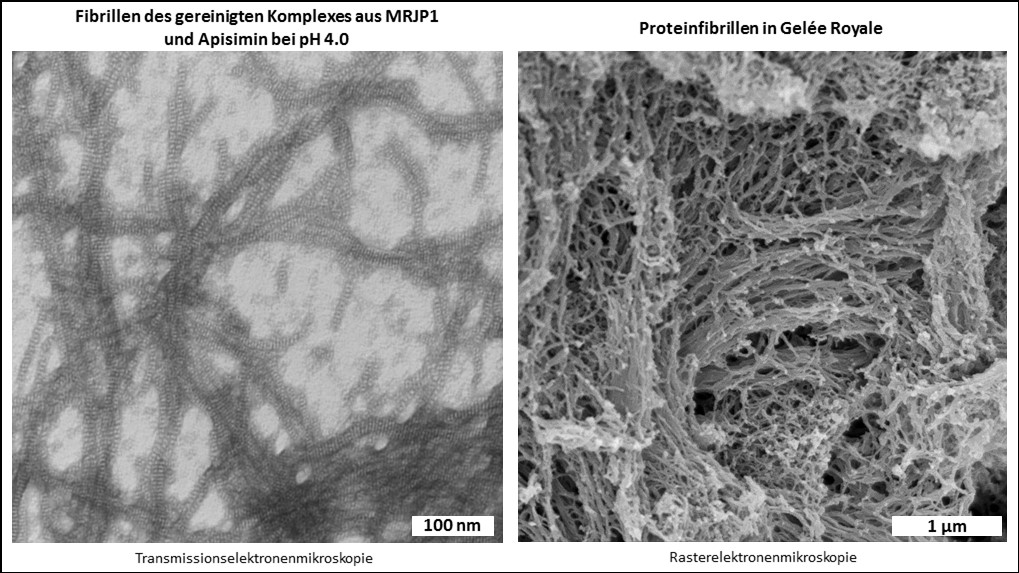
Nun stellte sich natürlich die Frage, was die Funktion dieser Proteinfibrillen ist, und ob der saure pH des Gelée Royales eigentlich wichtig ist?
Und natürlich ist er das! 🙂 Bei einem natürlichen pH von 4.0 hat Gelée Royale eine in etwa dreifach höhere Viskosität, als bei einem künstlich erhöhten pH von über 5.0 (Buttstedt et al., 2018). Das heißt, ist der pH zu hoch, wird Gelée Royale flüssiger. Und das hätte fatale Auswirkungen auf die Königinnenlarven (siehe Video), da diese in ihren Königinnenzellen sozusagen von der Decke hängen und mit dem Gelée Royale an der Decke festgeklebt sind. Somit sichern MRJP1 und Apisimin in Form der Fibrillen im Gelée Royale das Überleben des royalen Nachwuchses. Der niedrige pH des Gelée Royale wird durch Zugabe des Sekrets aus der Mandibeldrüse zum proteinhaltigen Sekret aus der Hypopharynxdrüse eingestellt. Dieses Sekret enthält viele freie Fettsäuren, vor allem 10-Hydroxy-2-decensäure (10-HDA), und die durch diese Fettsäure bedingte pH-Senkung des Futtersaftes bewirkt die Fibrillenbildung des Komplexes (Buttstedt, 2022).
Ein weiterer Vorteil des niedrigen Gelée Royale pHs ist, dass MRJP1, 2 und 3 bei saurem pH hitzestabiler
Ein weiterer Vorteil des niedrigen Gelée Royale pHs ist, dass MRJP1, 2 und 3 bei saurem pH hitzestabiler (Mureşan & Buttstedt, 2019) und deutlich resistenter gegenüber dem Abbau durch Proteasen sind (Mureşan et al., 2018), als bei neutralem pH.
Ein weiterer Bestandteil des Komplexes aus MRJP1 und Apisimin ist das Pflanzensterol 24-Methylenecholesterol (Tian et al., 2018). Während die Proteine MRJP1 und Apisimin, wie oben schon erwähnt, aus der Hypopharynxdrüse stammen, befindet sich 24-Methylenecholesterol zusammen mit den Fettsäuren im Mandibeldrüsensekret und wird so zum Futtersaft dazugegeben (Buttstedt et al., 2023). Dies bedeutet, dass sowohl der Proteinkomplex, als auch die Fibrillen erst nach dem Zusammenmixen von Hypopharynxdrüsen- und Mandibeldrüsensekret gebildet werden.